Chemotherapy Treatments
Inform yourself on the different types of chemotherapy.
Treatment Options
When you are diagnosed with glioblastoma, your healthcare team will work with you to choose the most appropriate treatment option. This often includes chemotherapy as a component of multimodal therapy, along with surgery, radiation therapy, and magnetic therapy.
Chemotherapy is the use of cytotoxic (cell killing) drugs to destroy tumor cells. Most cells in the body are constantly in a state of growth and/or division. However, tumor cells divide more rapidly than normal cells. Chemotherapy drugs often exploits this difference to target tumor cells for death by interfering with the way they divide, sparing the normal cells. Complications of most chemotherapy agents typically associate with cell division in normal cells, much as they interfere with cell division in the tumor cells.
There are several drugs that are FDA approved for use in malignant brain tumors including:
TEMODAR. American Journal of Neuroradiology September 2010, 31 (8) 1383-1384; DOI: https://doi.org/10.3174/ajnr.A2170
- Temozolomide (Temodar or TMZ)
- Lomustine (CCNU)
- Carmustine (BCNU or BiCNU)
- Avastin (bevacizumab)
On occasion, other drugs may be used ‘off-label’ for glioblastoma treatment. This means that the drug is not FDA approved for use in glioblastoma patients, but your physician feels there may be a benefit to using the drug. While this may seem troubling, many medications for all types of illness are used in off-label fashion to treat disease.
Another option for glioblastoma patients is to enroll in a clinical trial. Clinical trials test new drugs and procedures in patients to evaluate their safety and efficacy for specific diseases. This is a vital step in the development and FDA approval of new treatments.
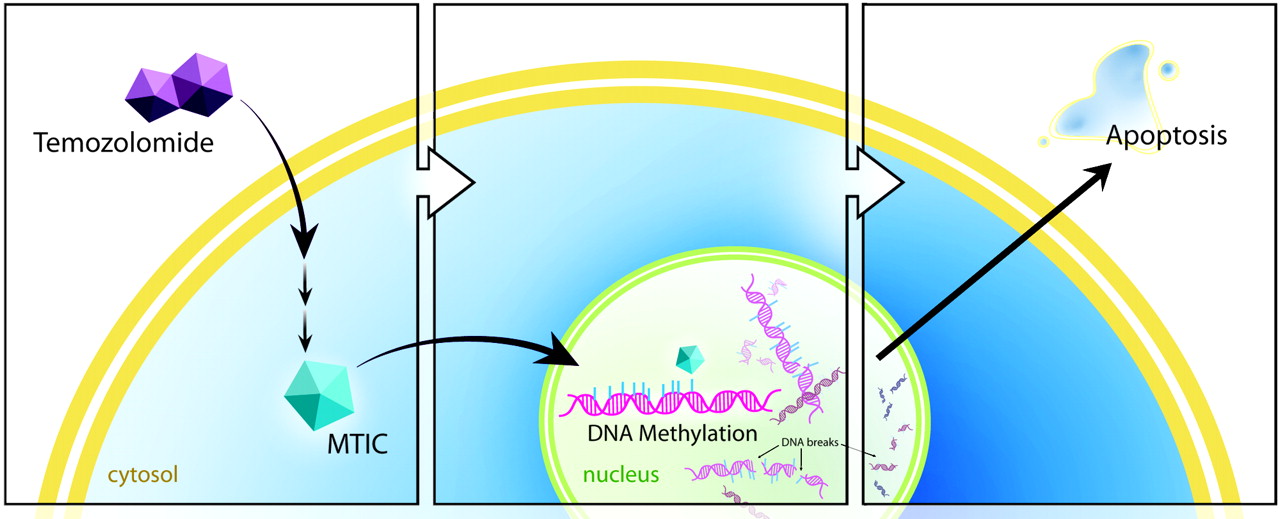
Temozolomide (Temodar, TMZ) is a chemotherapy drug that is used as part of the ‘standard of care’ for glioblastoma treatment. Standard of care refers to the recommended first line treatment for a disease provided by physicians. Most often there is supporting evidence to recommend such treatment before considering other options.
The standard of care for glioblastoma was developed over a decade ago and has remained largely unchanged since then. For glioblastoma, standard of care has 2 phases:
- Radiation Therapy and concomitant Temozolomide treatment:
- 4-6 Weeks after surgery, radiation therapy and Temozolomide treatment begins. This is known as the concomitant phase.
- Radiation is generally given for 6 weeks, 5 days a week, for a total of 30 treatments.
- During the 6 weeks of radiation treatment Temozolomide is taken once a day, every day. This is a total of 42 days of chemotherapy treatment.
- The dose of Temozolomide given depends on your height and weight. It is taken at home as one or more pills at the same time each day.
- Continued Temozolomide Treatment:
After a 4-week break from any treatments, the monotherapy or adjuvant phase begins. Temozolomide is taken for the first 5 days of a 28-day cycle. This is sometimes referred to as “5/23”: 5 days on, and 23 days off. A higher dose of Temozolomide is used in this phase, again dependent on height and weight. Treatment typically continues for 6 cycles.
Temozolomide is a type of chemotherapy drug known as an alkylating agent. Alkylating agents modify DNA by adding an alkyl group. This damages the DNA and interferes with cell growth and division, ultimately causing the death of the tumor cell.
All cells, whether tumor cells or normal cells, have mechanisms to repair damaged DNA and prevent cell death. One of the most important mechanisms working to repair DNA damage caused by Temozolomide involves an enzyme called MGMT. MGMT removes the alkylation caused by Temozolomide, allowing the tumor cells to survive.
In some people MGMT is in an inactivated form called “methylated MGMT”. In patients with this variation, Temozolomide is allowed to do its work without the DNA repair mechanisms saving the tumor cells from death. Having methylated MGMT has a favorable prognosis for the effectiveness of Temozolomide treatment.
Patients with unmethylated MGMT have a poorer prognosis, and Temozolomide is less likely to be effective in treating the tumor. However standard of care is commonly still used with these patients. This is because some percentage of cells may contain methylated MGMT, and even those with unmethylated MGMT can be susceptible to treatment. Patients with unmethylated MGMT may benefit from discussing the options for clinical trials with their physicians.
Currently, only a biopsy of the tumor sample can show what levels and form of MGMT is present in tumor cells.
Like most chemotherapy drugs, Temozolomide has a number of side effects that can range from mild to severe, and rare to common. What follows is not a full list of side effects and their severity. It is a guide to some of the most common issues patients face when taking Temozolomide, and some of the ways patients and their physicians can deal with them.
It is important that when taking Temozolomide that you follow the directions of your physician carefully, and seek medical care immediately if you have concerns.
During Temozolomide treatment you will have weekly blood tests to assess blood counts. Electrolytes and other labs will be obtained periodically also. These labs are important as they can alert your physician to blood or organ function problems that may require treatment to be paused.
Risk of infection:
Treatment with radiation, chemotherapy and steroids weaken a patient’s immune system and increase their risk of infection. An antibiotic, usually Bactrim, will generally be prescribed and taken 3 times a week during Temozolomide treatment to reduce the opportunistic infections that often associate with these treatments.
Nausea and vomiting:
These are common effects of Temozolomide treatment, but can be dealt with effectively using antiemetic medications. Generally, an antiemetic is taken 30 minutes to an hour before Temozolomide. There are several different antiemetics that your physician can prescribe, and if one does not seem to work to keep nausea at bay, others drugs can be tried.
Constipation:
Temozolomide treatment can cause constipation. It is important to eat a balanced diet and to drink plenty of fluids. You may want to have these over the counter medications on hand when starting Temozolomide treatment and talk with your physician about when and how to use them.
- Metamucil,
- Senokot
- Colace/Pericolace
Fatigue:
Radiation treatments are exhausting, and radiation to the brain is particularly fatiguing. The addition of chemotherapy can leave a patient with little energy and a whole-body tiredness that is not relieved by sleep. Eating a balanced diet that includes protein, and drinking plenty of fluids can help. It is important to get some gentle exercise along with plenty of rest and naps.
Appetite:
Some patients experience a decreased appetite, and food or smell aversions. This can make it difficult to eat and enjoy meals. Changing what you eat, having someone else cook outside to avoid smells, or eating more small snacks during the day can all help to alleviate these problems.
Reasons Temozolomide treatment may be paused or discontinued:
Throughout treatment with Temozolomide it is important to monitor blood counts and organ function via regular blood tests. It is not uncommon for problems to be revealed by these blood tests that may require treatment to be paused or even stopped. Common reasons for pausing or stopping treatment are:
- Absolute Neutrophil Count (White blood cells)
- Platelet Count (Ability to form clots)
- Myelosuppression (Bone marrow)
- Hepatotoxicity (Liver)
It is important to understand that there is currently no standard of care defined for recurrent glioblastoma, that is – glioblastoma that returns after an initial treatment. Potential treatments at this point may include repeat surgery, repeat radiation, additional chemotherapy, Avastin treatment or clinical trials.
Lomustine and Carmustine are two chemotherapy drugs known as nitrosoureas. They act in a very similar way to Temozolomide as they are also DNA alkylating agents. If a patient has unmethylated MGMT and has failed Temozolomide treatment, it is unlikely that the benefit of these types of drugs will outweigh problems caused by their side effects.
Lomustine and Carmustine are often combined with other medications such as Avastin, or clinical trial drugs. Lomustine is taken as an oral pill generally once every 6 weeks, and Carmustine is given as an IV infusion. Side effects for both drugs are similar to Temozolomide.
In both primary diagnosis and recurrent glioblastoma, BCNU wafers (Gliadel) are another option for direct chemotherapy treatment at the time of surgery. The drug effect is the same, but the drug is applied as an implant directly at the site of the tumor bed, thus increasing the concentration locally without the risk of systemic side effects. Patients typically require bolus steroids for about 30 days after surgery if Gliadel is applied to reduce the development of local edema caused by the drug. There have been some reports of increases in infection and cerebrospinal fluid leaks or wound issues associated with BCNU wafer implants. However, the benefit is an entire course of chemotherapy applied at one time. Another benefit is that the wafers are immediately active during a time when otherwise no therapy would be delivered (i.e. chemotherapy or radiation) in the immediate post-operative time period of 4-6 weeks.
Avastin (bevacizumab) has been FDA approved for use in recurrent glioblastoma. It is not a traditional chemotherapy drug as it does not directly attack and kill tumor cells. It is an anti-angiogenic drug, meaning it prevents the growth of new blood vessels. Avastin can be used alone or in combination with other drugs which are either off-label or as part of a clinical trial.
Avastin also reduces edema, or swelling in the brain. This can help to relieve symptoms caused by edema, and allow the reduction in the use of steroids by patients experiencing negative effects from edema.
Several studies have shown that when used alone, Avastin does not increase overall survival rates for patients. However, it does increase the time before disease progression is seen (Progression Free Survival) and can improve quality of life.
Avastin is given in a hospital or clinic as an IV infusion. The recommended dose when used alone is 10mg / kg every 2 weeks. However, this dose and schedule may be varied depending on the patient and if Avastin is being given alongside another drug.
The first infusion is given over 90 minutes, but as long as the drug is well tolerated the regular infusion time is 30 minutes. Patients can expect to provide a urine sample and have blood taken at each appointment to check blood counts and organ function. The results of these tests are needed before the infusion can begin.
Avastin treatment can continue for as long as the patient tolerates it, and for as long as it remains useful in controlling the tumor.
Avastin is a humanized monoclonal antibody that is designed to block a protein called VEGF, (vascular endothelial growth factor) which is important in the growth of new blood vessels. All cells produce VEGF, but some tumor cells produce too much of it. Blocking VEGF can prevent the growth of new blood vessels that the tumor needs to survive.
Like most drugs, Avastin has a number of side effects that can range from mild to severe, and rare to common. What follows is not a full list of side effects and their severity. It is a guide to some of the most common issues patients face when taking Avastin, and some of the ways patients and their physicians can deal with them.
It is important that when taking Avastin that you follow the directions of your physician carefully, and seek medical care immediately if you have concerns.
- GI perforation:
A hole that develops in your stomach or intestine. Symptoms include pain in your abdomen, nausea, vomiting, constipation, or fever. This is a serious yet rare side effect.
- High blood pressure:
Blood pressure will be monitored at each infusion appointment, and can be treated using blood pressure medications if necessary.
- Too much protein in the urine (Proteinuria):
This can be a sign of kidney damage and will be monitored using urine tests at each appointment. Protein in the urine is a reason that treatment may be delayed or paused.
- Bleeding or wounds that won’t heal:
Because Avastin decreases angiogenesis (vascular growth), it can cause slow or delayed wound healing and internal or external bleeding. This may be as mild as a nosebleed or more severe such as bleeding in the brain or stomach. Avastin should not be used for at least 28 days before or after surgery and until surgical wounds are fully healed.
Chemotherapy and Other Options in Glioblastoma Treatment
Chemotherapy is a cornerstone of glioblastoma treatment, along with surgical resection and radiation therapy. Glioblastoma standard of care has been essentially unaltered for over a decade, and as yet has not been improved upon. Clinical trials for glioblastoma are now focusing on targeted drugs, immunotherapy, and vaccine therapy. For a disease like glioblastoma where no cures are currently available, clinical trials are not just vital to research, but offer hope to patients.


